i3- structure hybridization|I3 : Manila Learn how to draw the Lewis structure of I3-, a triiodide ion with a negative charge, and how to calculate its hybridization, polarity, and molecular geometry. Find out the steps, formulas, and examples for this polyatomic . Russian girl with a big ass takes in her roommate's dick | Russian Amateur . Bad Hot Lady. 15.7K views. 89%. 54 years ago. 18:06. Roughly Fucked A Red-haired, Curvy Secretary For Bad Work. . Pornhub provides you with unlimited free porn videos with the hottest adult performers. Enjoy the largest amateur porn community on the net as well as .
PH0 · Lewis Dot of Triiodide Ion I3
PH1 · Lewis Dot Structure of I3
PH2 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
PH3 · I3 Lewis Structure, Molecular Geometry,
PH4 · I3
PH5 · I3
PH6 · Hybridization of Iodine in Triiodide ion (I3)
PH7 · Hybridization of I3(
GOLDEN BETTER LIFE. 41 – 3 – 16 – 7 – 67. 89 – 18 – 48 – 85 – 45. . Conclusion On Golden Chance Lotto Today. . yesterday and the previous games that have been played and predictions has also been given for today’s game. Related posts:
i3- structure hybridization*******Learn how to draw the Lewis structure of I3-, a triiodide ion with a negative charge, and how to calculate its hybridization, polarity, and molecular geometry. Find out the steps, formulas, and examples for this polyatomic .
It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the molecule. .
Learn how to determine the hybridization of I3-, a linear anion with sp3d hybridization, by using the formula or the lone pairs and valence electrons. See the Lewis .i3- structure hybridization I3 This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the . I3⁻ exhibits a linear geometry with bond angles of 180°, consistent with sp³d hybridization. The presence of the extra electron on the central iodine contributes to the ion’s . I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.Hybridization is a concept in molecular orbital theory that explains the mixing of atomic orbitals to form new, hybrid orbitals that are suitable for bonding in a molecule. In the case of I3−, the .
An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Triiodide Ion is also .
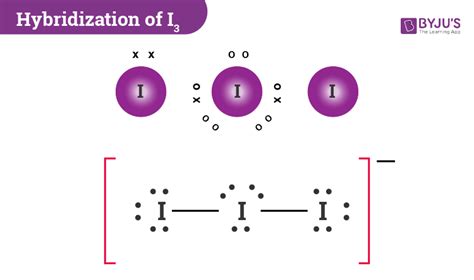
I 3- is dsp 3 hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Elements in the first 2 periods of the .Hybridization of I3- ion- The I3- ion is a polyatomic ion consisting of three iodine atoms bonded together with a negative charge.- The Lewis structure of I3- ion shows that it has a linear shape with the central iodine atom bonded to two terminal iodine atoms.- Hybridization is the process of combining atomic orbitals to form hybrid orbitals that are used to describe the bonding in . The Lewis structure of triiodide [I 3] – consists of three identical iodine (I) atoms. One I atom acts as the central atom while the other two iodine atoms act as outer atoms. There are a total of 5 electron density regions . This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and form.i3- structure hybridizationHybridization of I 3. The I 3 − molecule is known as a triiodide ion. It is an anion composed of three iodine atoms with a negative charge. 1.0 Hybridization of Triiodide Ion. Hybridization of I 3 − ion involves the iodine atoms, which exhibit a form of hybridization that leads to the linear molecular structure of the ion. Hybridization is a concept in molecular orbital theory that . Prediction of sp, sp2, sp3 Hybridization state; Prediction of sp3d, sp3d2, and sp3d3 Hybridization States; References; External Links; Contributor; Prof. Linus Pauling (1931) first developed the Hybridization state theory in order to explain the structure of molecules such as methane (CH 4). 1 This concept was developed for simple chemical systems but this one .
H= Hybridization V= Number of Valence electrons C= Charge on cation or more electropositive element. A= Charge on anion or more electronegative element. Now if H =2, then it shows Sp hybridization. H= 3, it represents Sp2 hybridization. H= 4, it will show Sp3 hybridization. H=5 means it isSp3d hybridized. H=6, the molecule will have Sp3d2 . I3- ion has sp3d hybridization and linear shape containing two bond pair and three lone pair while I3+ ion has sp3 hybridization and bent shape containing two bond pair and two lone pair. . The structure of crystalline solids is determined by packing of their constituents .In order to understand the packing of the constituen.
Geometrical isomers. For some molecules in the Table, we note that there is more than one possible shape that would satisfy the VSEPR rules. For example, the XeF 2 molecule has a steric number of five and a trigonal bipyramidal geometry. There are three possible stereoisomers: one in which the F atoms occupy axial sites, resulting in linear molecule, one in .Hybridization of ClF3 - Chlorine Trifluoride is sp3d hybridized. Understand the structure, shape and Hybridization of ClF3. Determine the hybridization of Cl in ClF3.
#Hybridization of (I)3- , CHEMICAL BONDING#I3-,(I)3-#studyBibhaschemical bonding class 11,chemical bonding video lecture,hybridisation class 11 iit jee,how t.Hybridization-to find the no.of hybridized bonds, add the no. of lone pairs with the no.of neighbour atoms. 3 l . p + 2 ( i o d i n e a t o m s ) = 5 So Hybridization of l 3 − = s p 3 d An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.
Hybridization of SF6. Lewis structure is a representation of bond formation. But if you need to understand how a compound looks on a plane then we need to find its hybridization and further molecular geometry. Here is a .Groups are placed around the central atom in a way that produces a molecular structure with the lowest energy, that is, the one that minimizes repulsions. In the VSEPR model, the molecule or polyatomic ion is often given an AX m E n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (usually a .In chemistry, triiodide usually refers to the triiodide ion, I − 3.This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine.Some salts of the anion have been isolated, including thallium(I) triiodide (Tl + [I 3] −) and ammonium triiodide ([NH 4] + [I 3] −).Triiodide is observed to be a red colour in . Nitrogen triiodide (NI3) lewis dot structure, molecular geometry, hybridization, polarity. Nitrogen triiodide appears as purple gas is an inorganic compound having the chemical formula NI3. It releases a purple cloud of iodine vapor when touched gently because of its extremely sensitive contact explosion property. .

What is the Hybridization of Xenon Difluoride? In the hybridization of xenon difluoride, Xenon (Xe) is the central atom. Now if we count the number of valence shell in Xe we will find two electrons in the 5s orbital and six electrons in the 5p orbital.What is the Hybridization of Sulphur Tetrafluoride? In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons.
パチンコパチスロの可能性を信じる動画を配信する為にいざ集結。SEVEN’S TV (セブンズティービー) 公式チャンネルです。「SEVEN’S 」オンライン .
i3- structure hybridization|I3